UltraPlasma™ Hidradenitis Suppurativa Treatment
withOut! Drugs, Industrial Chemicals, Medicines, Surgery, Supplements, and Lasers.
TREATMENTSAESTHETICSBEAUTY
MedicaLabs, Ltd. | https://medicalabs.com
5/8/202410 min read
Abstract
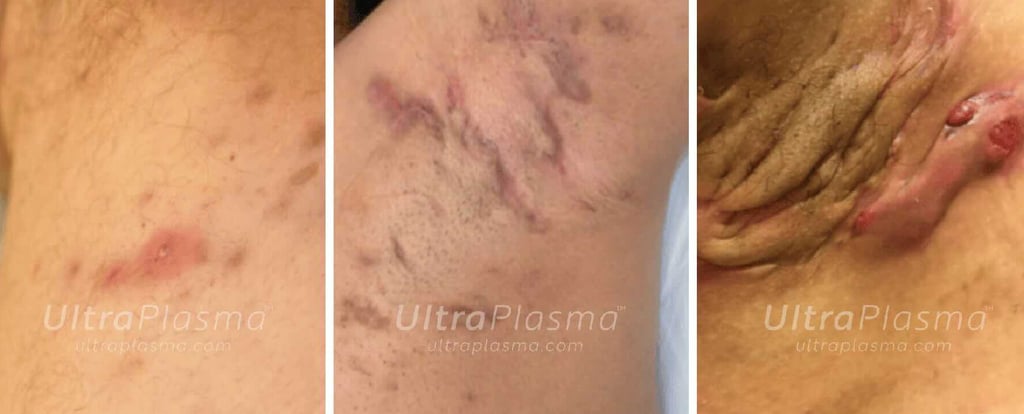
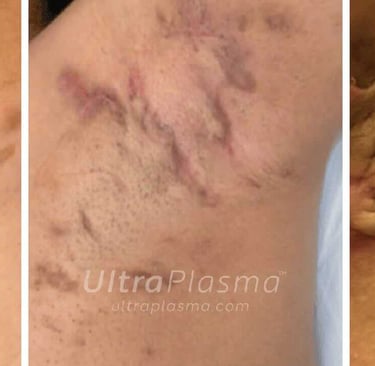
Hidradenitis Suppurativa (HS)—also known as Acne Inversa—is a debilitating chronic inflammatory skin disorder primarily affecting the pilosebaceous-apocrine unit in intertriginous areas such as the axillae, groin, and perineum. The pathogenesis of HS is multifactorial, involving follicular occlusion, immune dysregulation, genetic predisposition, microbiome alteration, and mechanical stress. Characterized by painful nodules, recurrent abscesses, sinus tract formation, and dermal fibrosis, HS significantly impairs quality of life and is often resistant to conventional treatments including antibiotics, immunosuppressants, and surgical interventions.
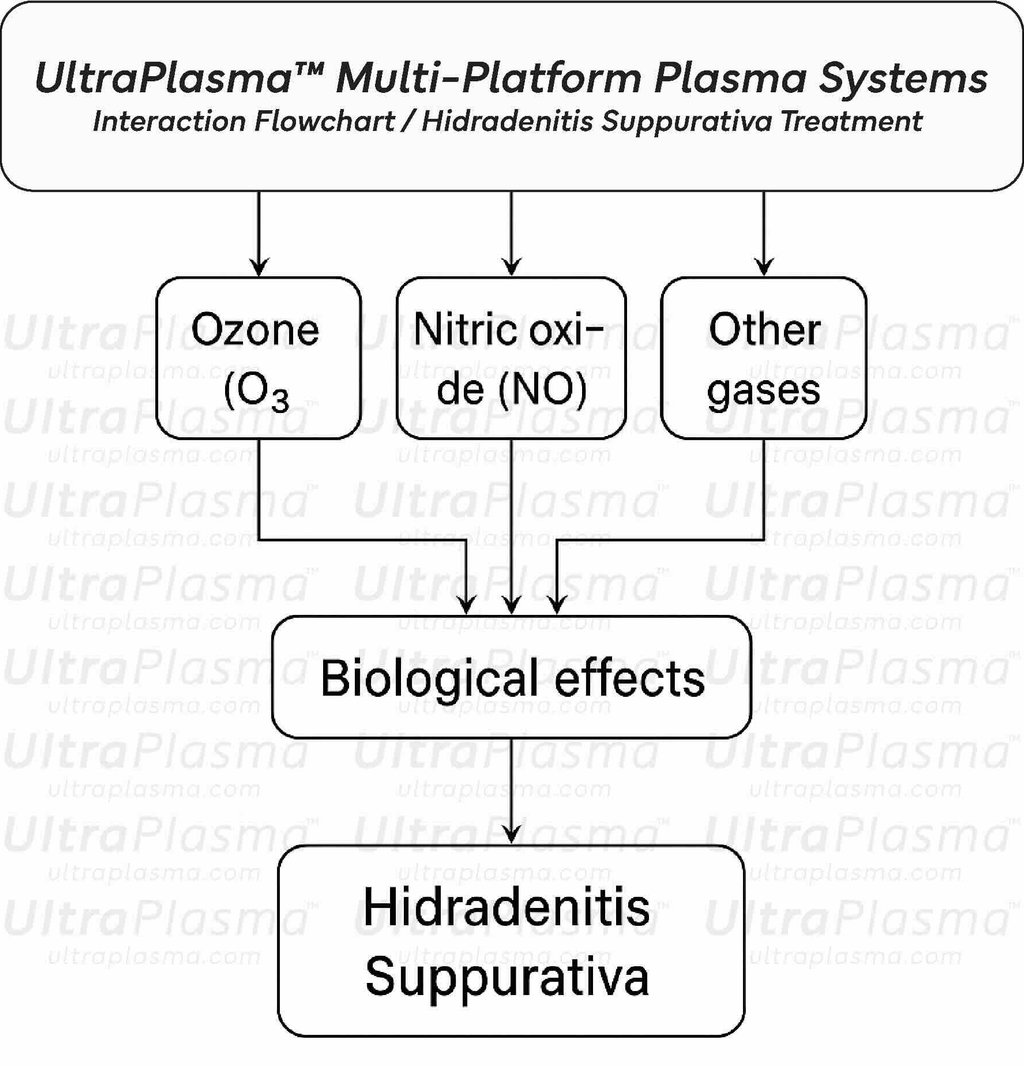
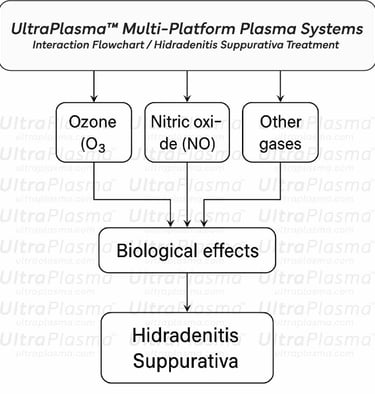
Recent advances in plasma medicine have introduced UltraPlasma™ multi-platform plasma systems as a novel, minimally invasive therapeutic modality. Utilizing arc, argon, and helium plasma emissions, UltraPlasma™ targets pathological features of HS at distinct skin depths—epidermis, dermis, and hypodermis—via localized thermal, chemical, and electrical bio-interactions. These effects are mediated by reactive gases such as ozone (O₃), nitric oxide (NO), and other reactive oxygen and nitrogen species (RONS), leading to microbial sterilization, immune modulation, angiogenesis, and extracellular matrix remodeling.
This article explores the anatomical, immunological, and engineering foundations of HS and examines how UltraPlasma™ addresses its core mechanisms through layered, gas-assisted plasma penetration. Supported by histological data and clinical imagery, this integrated approach may offer a paradigm shift in the treatment of HS by providing localized control of inflammation, infection, and scarring with reduced recurrence and enhanced skin regeneration.
1. Introduction
Hidradenitis Suppurativa (HS), also known as Acne Inversa, is a chronic, inflammatory, and recurrent skin disease characterized by painful nodules, abscesses, sinus tracts, and scarring. Typically affecting the apocrine gland-bearing areas such as the axillae, groin, and perianal regions, HS poses significant treatment challenges due to its deep dermal and hypodermal involvement. The UltraPlasma™ system introduces an innovative, non-invasive therapeutic strategy utilizing multi-platform plasma technology—arc, argon, and helium plasmas—to target pathophysiological layers with gas-mediated bioactivation.
2. Anatomical and Pathological Basis of Hidradenitis Suppurativa
2.1 Affected Skin Layers
Epidermis: Hyperkeratosis and follicular occlusion initiate HS.
Dermis: Inflammation spreads, forming painful nodules and pus.
Hypodermis: Chronic progression leads to tunnel formation, fibrosis, and deep abscesses.
2.2 Immunological and Microbiological Dynamics
Overexpression of TNF-α, IL-1β, and IL-17.
Colonization by Staphylococcus aureus, anaerobes, and biofilm-forming bacteria.
Dysregulation in innate immune response and keratinocyte signaling.
3. UltraPlasma™ Technology Overview
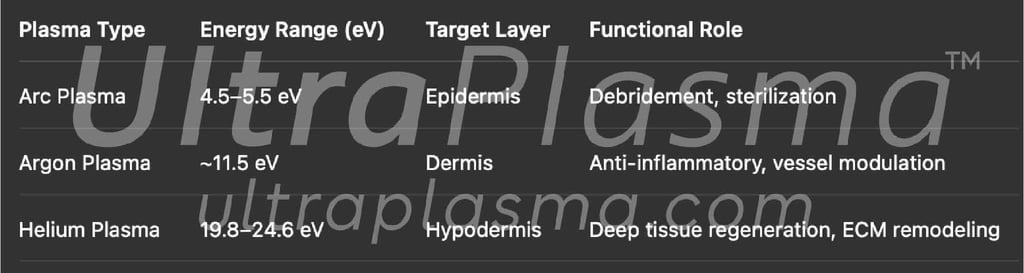
3.1 Plasma Modalities and Energy Profiles


4. Plasma-Gas Bio-Interactions in Hidradenitis Suppurativa (HS) Treatment
The therapeutic action of UltraPlasma™ in Hidradenitis Suppurativa is driven by its unique ability to initiate controlled plasma-gas interactions at precise skin depths. These interactions generate reactive oxygen and nitrogen species (RONS)—including ozone (O₃), nitric oxide (NO), hydrogen peroxide (H₂O₂), peroxynitrite (ONOO⁻), and hydroxyl radicals (•OH)—each playing distinct biological roles in antimicrobial defense, immune modulation, and tissue remodeling.
4.1 Ozone (O₃) – Epidermal Sterilization and Oxygenation
Mechanism of Action:
Generated from oxygen in ambient air or injected O₂ during arc plasma emission.
Reacts rapidly with bacterial cell membranes, biofilm matrices, and oxidizable lipids.
Increases tissue oxygen tension and perfusion at the wound margin.
HS-Specific Effects:
Disrupts colonization by Staphylococcus aureus, Corynebacterium, and anaerobes often implicated in HS lesions.
Decomposes sebaceous and follicular debris contributing to follicular occlusion.
Enhances re-epithelialization of ulcerated areas.
4.2 Nitric Oxide (NO) – Immune Modulation and Vasodilation
Mechanism of Action:
Produced through plasma-induced breakdown of nitrate/nitrite reservoirs or directly from nitrogen-containing gases.
Diffuses into dermal microvasculature and immune cell populations.
HS-Specific Effects:
Induces vasodilation → improves perfusion in chronically inflamed, ischemic tissues.
Inhibits overexpression of pro-inflammatory cytokines like TNF-α, IL-1β, IL-17, which are elevated in HS.
Promotes M2 macrophage polarization, reducing destructive neutrophilic activity.
4.3 Reactive Oxygen and Nitrogen Species (RONS) – Remodeling and Regeneration
Mechanism of Action:
High-energy arc and helium plasmas produce •OH, ONOO⁻, singlet oxygen (¹O₂), and superoxide (O₂⁻).
These species exert selective oxidative stress that destroys damaged cells and stimulates adaptive tissue responses.
HS-Specific Effects:
Triggers matrix metalloproteinases (MMPs) → collagen breakdown in fibrotic sinus tracts.
Activates fibroblasts and keratinocytes for tissue remodeling and re-epithelialization.
Induces heat-shock proteins (e.g., HSP70, HSP90), initiating cellular repair and anti-inflammatory cascades.
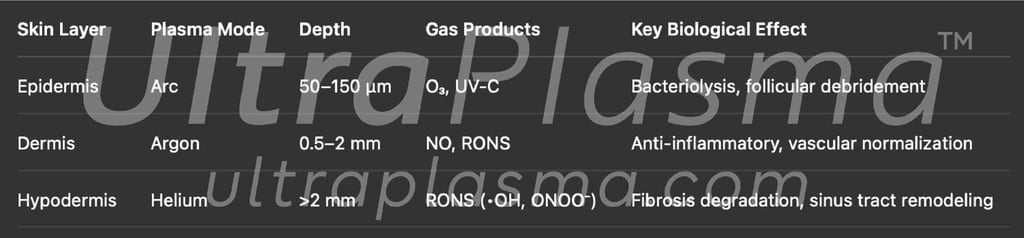
4.4 Plasma-Gas Depth Mapping in HS


⌘Discussion⌘
Hidradenitis Suppurativa (HS) remains one of the most challenging chronic dermatological diseases to treat, primarily due to its multifactorial pathology and layered tissue involvement. Traditional treatment modalities—including long-term antibiotics, immunomodulators, corticosteroid injections, and even surgical excisions—often fail to provide sustained remission or carry high recurrence and morbidity rates. In this context, the emergence of plasma medicine, particularly via the UltraPlasma™ multi-platform system, offers a paradigm shift in the management of HS.
The UltraPlasma™ approach is fundamentally distinct in its ability to selectively target skin layers—epidermis, dermis, and hypodermis—through different plasma types (arc, argon, and helium) while delivering biochemically active gases such as ozone (O₃), nitric oxide (NO), and reactive oxygen/nitrogen species (RONS). This cross-sectional and gas-mediated specificity allows UltraPlasma™ not only to eliminate surface pathogens and biofilms but also to modulate inflammation, restore vascular function, and remodel subdermal fibrotic tissues that are hallmarks of advanced HS.
Our anatomical analysis showed that arc plasma is highly effective at resolving superficial follicular occlusion and sterilizing inflamed epidermal surfaces, a critical step in halting the early triggers of HS. Meanwhile, argon plasma’s NO-mediated immune modulation appears to directly address the cytokine-driven chronic inflammation in the dermis—a pathophysiological process often refractory to systemic medications. Furthermore, helium plasma demonstrates deep tissue penetration and regenerative potential, capable of accessing and remodeling sinus tracts and subcutaneous fibrotic tunnels—structures traditionally considered irreversible without surgical intervention.
In terms of gas interaction dynamics, the synergy of ozone and RONS enhances both antimicrobial clearance and tissue regeneration, while NO plays a key role in angiogenesis, immune balance, and pain modulation. This biochemical interplay, guided by plasma energy delivery, mimics a biologically intelligent healing cascade without pharmacological burden or systemic toxicity.
From an engineering perspective, the real-time feedback, thermal regulation, and gas control systems embedded in UltraPlasma™ ensure high safety and reproducibility. The integration of multi-gas microchannels, proximity sensors, and plasma depth modulation algorithms allows clinicians to fine-tune treatment according to lesion severity and location. These features are especially relevant in sensitive, high-friction anatomical zones typical of HS (axillae, groin, inframammary areas), where precision and safety are paramount.
Despite its promise, limitations remain. Plasma therapy for HS is still a novel field, and long-term, large-scale clinical trials are needed to validate durability, recurrence rates, and cost-effectiveness. Variability in gas interaction profiles due to patient-specific skin biochemistry, and technical challenges in standardizing energy delivery in tunnels with variable morphology, require further refinement. Moreover, combination protocols with biologics or surgical de-roofing procedures may be warranted in complex or refractory cases.
Nevertheless, the multi-dimensional capability of UltraPlasma™—to simultaneously decontaminate, immunomodulate, and remodel tissue—offers an integrative solution unmatched by conventional approaches. Its modular platform also enables adaptability to different lesion types and disease stages, potentially reducing the need for long-term systemic therapy and improving patients’ quality of life.
5. Cross-Sectional Treatment Mechanism
The pathology of Hidradenitis Suppurativa (HS) spans multiple layers of the skin—epidermis, dermis, and hypodermis—requiring a layered, precision-based therapeutic strategy. UltraPlasma™’s multi-platform plasma technology is uniquely suited for this task, delivering arc, argon, and helium plasma types selectively to specific depths. This cross-sectional application ensures maximal therapeutic effect with minimal collateral damage.
5.1 Epidermal Layer (Arc Plasma – Superficial Debridement & Sterilization)
Depth: 50–150 µm
Primary Issues:
Follicular occlusion by keratin plugs.
Surface colonization by pathogenic bacteria and biofilms.
Disrupted skin barrier function.
UltraPlasma™ Effect:
Arc plasma (4.5–5.5 eV) delivers focused superficial energy, ideal for epidermal interaction.
Produces ozone (O₃) and UV-C radiation, destroying bacterial colonies and exfoliating occluded follicles.
Stimulates keratinocyte turnover and promotes barrier recovery without significant thermal damage.
Result:
Cleared follicular openings, decreased microbial load, and initiation of re-epithelialization.
5.2 Dermal Layer (Argon Plasma – Anti-Inflammatory and Vascular Modulation)
Depth: 0.5–2 mm
Primary Issues:
Persistent inflammation with cytokine imbalance (↑ TNF-α, IL-1β, IL-17).
Dilated capillaries, edema, and tissue ischemia.
Formation of painful nodules and tunnel precursors.
UltraPlasma™ Effect:
Argon plasma (~11.5 eV) penetrates mid-dermal tissues.
Produces nitric oxide (NO) and low-level RONS that:
Normalize vascular tone and perfusion via vasodilation.
Suppress pro-inflammatory signaling.
Promote macrophage phenotype shift (M1 → M2).
Result:
Reduced dermal inflammation, improved oxygenation, and preemptive interruption of abscess and tract formation.
5.3 Hypodermal Layer (Helium Plasma – Sinus Tract Remodeling and Fibrosis Breakdown)
Depth: >2 mm
Primary Issues:
Chronic abscesses and epithelialized sinus tracts.
Fibrotic tissue bridges and contractile scarring.
Entrapped debris and immune-resistant infection zones.
UltraPlasma™ Effect:
Helium plasma (19.8–24.6 eV) delivers high-energy photons into the hypodermis with deep gaseous diffusion.
Induces intense RONS activity (•OH, ONOO⁻, ¹O₂) that:
Break down extracellular matrix fibrosis via MMP stimulation.
Promote neoangiogenesis and fibroblast recruitment.
Support tunnel sealing and dermal regeneration.
Result:
Reduction of sinus tracts, softened fibrosis, restoration of subcutaneous tissue architecture.
5.4 Integrated Layered Response – Summary Table
6. Engineering Aspects of UltraPlasma™ for HS
6.1 Pulse Control & Depth Modulation
Adjustable waveform emissions allow selective targeting (50–500 µm epidermal, 0.5–2 mm dermal, >2 mm hypodermal).
6.2 Integrated Gas Flow Channels
Controlled O₂/He/Ar gas infusion to optimize plasma stability and reactive species delivery.
6.3 Thermal and Electrical Safety Management
Non-contact applicators reduce cross-contamination and enable application over sinus openings.



⌘Conclusion⌘
Hidradenitis Suppurativa (HS) represents a complex and multifactorial dermatological disorder that defies conventional single-layered treatment strategies. Its progression through epidermal follicular occlusion, dermal inflammation, and hypodermal sinus formation necessitates a therapeutic approach that is both multi-depth and multifunctional. The UltraPlasma™ multi-platform system offers a unique and innovative modality that integrates arc, argon, and helium plasma energies with reactive gas therapeutics, enabling precise engagement with the pathophysiological processes at each skin layer.
By utilizing plasma-generated ozone (O₃), nitric oxide (NO), and reactive oxygen/nitrogen species (RONS), UltraPlasma™ facilitates localized antimicrobial action, immune modulation, tissue oxygenation, and extracellular matrix remodeling. This creates a biologically intelligent healing cascade that addresses HS not only at its visible symptoms but at its systemic biochemical roots. Moreover, the engineering backbone of the device—featuring real-time thermal regulation, gas control, and depth-targeting algorithms—ensures safety, reproducibility, and adaptability across varied anatomical regions and HS stages.
The integration of gas-assisted biointervention with precision engineering may signal a transformative step in HS management, potentially reducing the dependency on systemic pharmacologics, minimizing surgical interventions, and improving patient quality of life. Future studies should focus on long-term efficacy, combination protocols, and the role of UltraPlasma™ in preventative care for early-stage HS.
In summary, UltraPlasma™ represents a next-generation, cross-sectional solution for a historically intractable disease—uniting the fields of plasma physics, dermatological immunology, and regenerative medicine into a single therapeutic framework.
Advanced Plasma-Based Treatment of Hidradenitis Suppurativa Using UltraPlasma™ Multi-Platform Arc, Argon, and Helium Systems
3.2 Wavelength and Energy Effects on Hidradenitis Suppurativa (HS)
The therapeutic efficacy of UltraPlasma™ in treating Hidradenitis Suppurativa (HS) is largely governed by the plasma’s electromagnetic wavelength and electron volt (eV) energy output, which directly influence depth of penetration, biological specificity, and gas-molecule interaction efficiency in skin tissue.
Each plasma mode within the UltraPlasma™ system—arc, argon, and helium—operates in a distinct wavelength-energy profile, enabling targeted treatment across skin layers implicated in HS pathology.
3.3 Gas Delivery and Engineering Control Systems
The effectiveness of the UltraPlasma™ system in managing Hidradenitis Suppurativa (HS) relies not only on its energy profile and plasma wavelength but also on its precise gas delivery infrastructure and real-time engineering control systems. These systems ensure stable plasma generation, targeted reactive gas release, and safe, localized treatment of deeply infected and fibrotic tissues.
3.3.1 Gas Input Channels and Flow Regulation
Each UltraPlasma™ handpiece is engineered with dual or triple-lumen microchannels, allowing for the independent or mixed delivery of:
Argon (Ar): An inert carrier gas used to stabilize plasma discharge and promote smooth dermal penetration.
Helium (He): Facilitates deeper plasma diffusion into hypodermal layers due to its low atomic mass and high thermal conductivity.
Oxygen (O₂) or ambient air: Generates ozone (O₃) and RONS upon plasma excitation, enhancing antimicrobial and remodeling effects.
Gas flow rates are programmable in the 0.1–3.0 L/min range, allowing the clinician to tailor treatment intensity by anatomical location and Hurley stage of HS.
3.3.2 Onboard Microprocessor-Controlled Feedback Loops
The UltraPlasma™ system integrates real-time feedback sensors and a microcontroller (e.g., STM32-class MCU) that monitor and adjust:
Plasma stability (arc voltage, frequency modulation)
Skin-surface temperature (thermal cutoff < 45°C)
Distance calibration between applicator and skin (infrared proximity sensors)
Gas pressure and purity levels
These feedback mechanisms ensure uniform energy application and prevent overtreatment, a key requirement in sensitive HS regions such as axillae and groin folds.
3.3.3 Smart Mode Switching (Arc ↔ Argon ↔ Helium)
UltraPlasma™ incorporates automatic plasma mode switching based on:
Depth mapping algorithms (via dermal impedance or ultrasound feedback)
Operator preset profiles (e.g., “Early-Stage Nodule” vs “Chronic Tract” mode)
Gas composition ratios (e.g., >70% helium triggers helium-mode auto-initiation)
This dynamic response ensures that arc plasma focuses on superficial debridement, while argon and helium penetrate progressively deeper, reaching the sinus tracts and fibrotic bridges of advanced HS.
3.3.4 Plasma-Skin Contact Geometry
The UltraPlasma™ applicator tip features:
A non-contact plasma cone design to avoid mechanical pressure on inflamed tissue.
Precision-directed nozzles for linear or radial gas-plasma shaping.
Replaceable biocompatible shields (e.g., PTFE) to prevent contamination during sinus entry.
This is particularly beneficial in treating open HS tunnels, where conventional contact-based tools pose infection risks or disrupt fragile healing tissue.
3.3.5 Energy Consumption and Power Design
Total device power rating: 20–40 W, depending on plasma type and gas flow.
Plasma ignition frequency: 20–60 kHz pulse-modulated, minimizing arcing risk.
Safety class: EN 60601-1 compliant, suitable for both outpatient dermatology and minor surgical settings.
In summary, the gas delivery and engineering control systems of UltraPlasma™ are tailored to the anatomical, thermal, and biochemical needs of HS therapy. Through multi-gas integration, microprocessor feedback, and mode-switching algorithms, UltraPlasma™ delivers precision-controlled plasma exposure, ensuring safe and effective outcomes even in complex and chronic HS presentations.

4.5 Synergistic Effects in Inflammatory Microenvironments
In HS, the dermal microenvironment is characterized by:
Neutrophil infiltration and extracellular traps (NETs),
Biofilm-protected pathogens,
Oxidative imbalances and hypoxia.
Plasma-gas synergy counteracts these by:
Breaking down NETs via peroxynitrite and superoxide radicals.
Inhibiting microbial quorum sensing, thereby blocking biofilm expansion.
Restoring oxidative equilibrium, promoting regenerative signaling instead of chronic inflammation.
5.5 Clinical Implications
The cross-sectional strategy allows clinicians to:
Customize treatment depth per lesion stage (e.g., superficial nodules vs deep tunnels).
Avoid overtreatment of uninvolved tissues.
Achieve rapid symptom relief, lesion clearance, and long-term structural restoration.
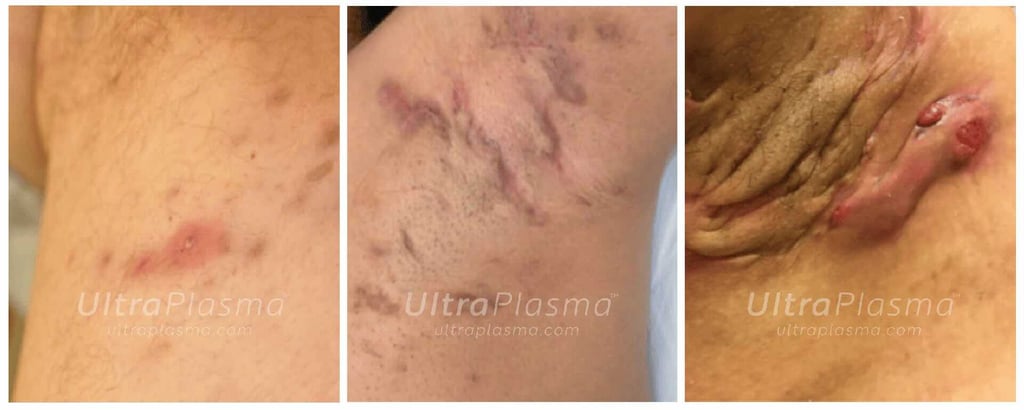
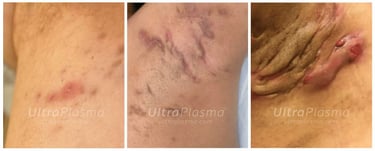
7. Clinical Outcomes and Case Evidence
8. Advantages over Conventional Therapies




Shortcut for our Goals!
▼


© 2021-2026. All rights reserved.
MedicaLabs Healthcare Technologies Ltd.
https://medicalabs.com


worldwide ultraplasma.com
countries locally - coming soon:
ultraplasma.de | ultraplasma.us | ultraplasma.cn | ultraplasma.uk | ultraplasma.it | ultraplasma.fr | ultraplasma.tr | ultraplasma.co.il | ultraplasma.az | ultraplasma.ru | ultraplasma.kr | ultraplasma.es | ultraplasma.in | ultraplasma.gr | ultraplasma.cz | ultraplasma.se | ultraplasma.cl | ultraplasma.rs | ultraplasma.sg | ultraplasma.qa | ultraplasma.nl | ultraplasma.dk | ultraplasma.ro | ultraplasma.fi | ultraplasma.co.za | ultraplasma.pt | ultraplasma.al | ultraplasma.pk | ultraplasma.si | ultraplasma.ch | ultraplasma.at | ultraplasma.lt | ultraplasma.nz | ultraplasma.ae | ultraplasma.hu | ultraplasma.pl | ultraplasma.be | ultraplasma.am | ultraplasma.ar | ultraplasma.uz | ultraplasma.io | ultraplasma.info | ultraplasma.ai
